

Most of them are used as fuel in vehicles. Dimethyl ether is a substance that can be used as a substitute for liquefied petroleum gas (LPG) because of its similar physical properties. To find the best alternative energy to replace in the future. Currently, research and research on alternative energy is ongoing. In addition, oil and gas are limited and likely to run out in the future. At present, prices of primary energy sources such as oil and natural gas tend to increase. The consumption of energy is increasing continuously due to the economic expansion of the world fleet. The most important and most used energy sources are crude oil and natural gas. This is due to the growth of industry and transportation. doi: 10.3390/pr7010054.Teerapat Laiwatthanaphaisarn and Amata Anantpinijwatna *ĭepartment of Chemical Engineering, Faculty of Engineering, King Mongkut’s Institute of Technology Ladkrabang, Bangkok, ThailandĬorresponding author: world energy consumption is likely to increase over time. Pilot Plant Data Assessment in Anaerobic Digestion of Organic Fraction of Municipal Waste Solids. Migliori M., Catizzone E., Giordano G., Le Pera A., Sellaro M., Lista A., Zanardi G., Zoia L. What a Waste: A Global Review of Solid Waste Management.
Waste management, waste resource facilities and waste conversion processes. Carbon dioxide recycling: Emerging large-scale technologies with industrial potential. Quadrelli E.A., Centi G., Duplan J.L., Perathoner S. Globalization and carbon emissions: Is there any role of agriculture value-added, financial development, and natural resource rent in the aftermath of COP21? J. The final environmental impact was found equal to -113 kgCO 2/GJ, demonstrating that DME synthesis from digestate may be considered as a suitable strategy for carbon dioxide recycling.Ĭarbon footprint digestate dimethyl ether gasification process simulation. Results show direct DME synthesis global yield was higher without the WGS section and with a carbon capture equal to 85%.

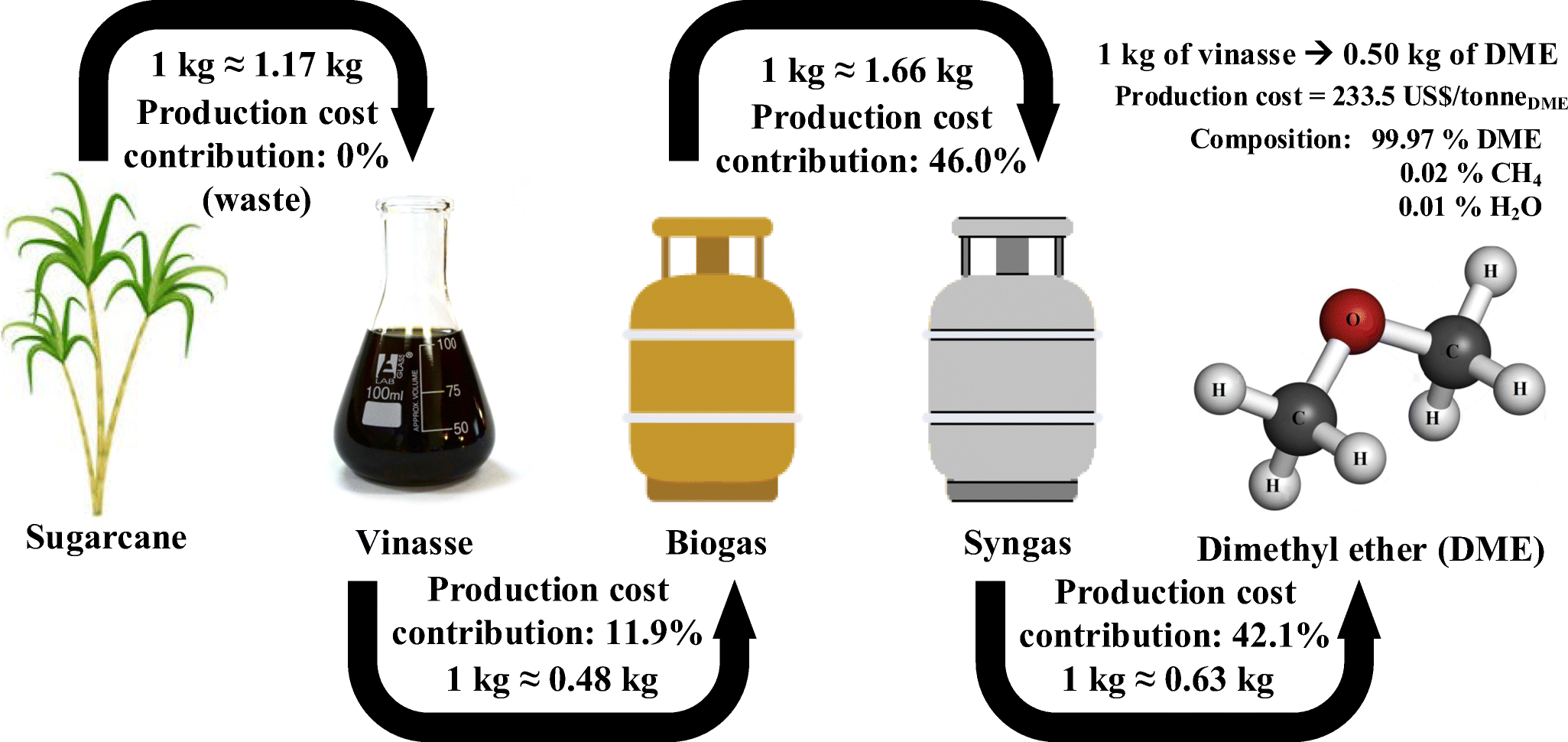
The final best flowsheet and the best process conditions were evaluated in terms of CO 2 equivalent emissions. Process simulation was carried out by ChemCAD software, and it was particularly focused on the effect of process conditions of both water gas shift and CO 2 absorption by Selexol ® on the syngas composition, with a direct influence on DME productivity. In particular, a thermodynamic analysis was performed to individuate the best process conditions and syngas conditioning processes to maximize yield to dimethyl etehr (DME). In this work, we simulate the synthesis of dimethyl ether from a syngas (a mixture of CO, CO 2 and H 2) produced from gasification of digestate. The production of dimethyl ether from renewables or waste is a promising strategy to push towards a sustainable energy transition of alternative eco-friendly diesel fuel.


 0 kommentar(er)
0 kommentar(er)
